32
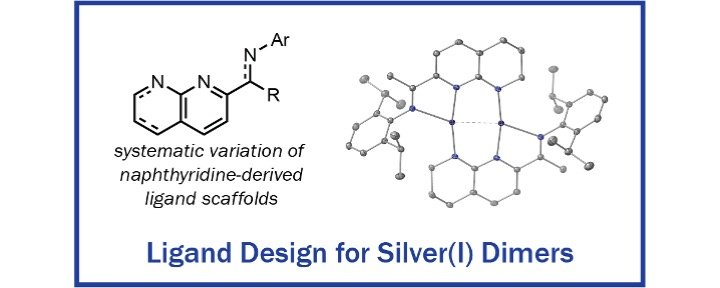
A Systematic Study of Naphthyridine-Derived Ligands for Well-Defined Silver(I) Dimers
S.T. Bavaresco, N.P. Marquard, E. Miura-Stempel, M.G. Mohsen, M.G. Campbell
Dalton Trans. 2025, 54, 13651–13657.
31
Integrating fundamental concepts with practical skills: consolidating small-molecule crystallography education
S.-L. Zheng, N.P. Litak, M.G. Campbell, R.C. Handford, D.K. Dilek, K.M. Carsch, T.A. Betley
J. Appl. Cryst. 2025, 58, 269–275.
30
Determination of Arsenic Content in Water Using a Silver Coordination Polymer
N.D. Reich, A.A. Nghiem, S. Nicholas, B.C. Bostick, M.G. Campbell
ACS Environ. Au 2022, 2, 150–155.
29
Teaching space group diagrams to chemistry students through a peer tutoring approach
S.-L. Zheng, M.G. Campbell
Acta. Cryst. 2021, E77, 864–866.
Part of a special issue on "modern approaches and tools for teaching crystallography," as well as a tribute to the late Jerry P. Jasinski.
28
Dinuclear Silver Complexes in Catalysis
T. Elkoush, N.D. Reich, M.G. Campbell
Angew. Chem. Int. Ed. 2021, 60, 22614–22622.
27
Visible Light Absorption and Long-Lived Excited States in Dinuclear Silver(I) Complexes with Redox-Active Ligands
D.J. Shields, T. Elkoush, E. Miura-Stempel, C.L. Mak, G.-H. Niu, A.D. Gudmundsdottir, M.G. Campbell
Inorg. Chem. 2020, 59, 18338–18344.
26
Silver(II) and Silver(III) Intermediates in Alkene Aziridination with a Dinuclear Silver(I) Nitrene Transfer Catalyst
T. Elkoush, C.L. Mak, D.W. Paley, M.G. Campbell
ACS Catal. 2020, 10, 4820–4826.
25
Bimetallic Photoredox Catalysis: Visible Light-Promoted Aerobic Hydroxylation of Arylboronic Acids with a Dirhodium(II) Catalyst
H.-M. Yang, M.-L. Liu, J.-W. Tu, E. Miura-Stempel, M.G. Campbell, G.J. Chuang
J. Org. Chem. 2020, 85, 2040–2047.
24
Switchable Electrical Conductivity in a Three-Dimensional Metal–Organic Framework via Reversible Ligand n-Doping
H.C. Wentz, G. Skorupskii, A.B. Bonfim, J.L. Mancuso, C.H. Hendon, E.H. Oriel, G.T. Sazama, M.G. Campbell
Chem. Sci. 2020, 11, 1342–1346.
Part of the Chemical Science themed collection “Most popular 2019-2020 supramolecular chemistry articles”
23
Silver(I) Coordination Polymers from Dinucleating Naphthyridine Ligands
G.-H. Niu, H.C. Wentz, S.-L. Zheng, M.G. Campbell
Inorg. Chem. Commun. 2019, 101, 142–144.
22
Connecting Key Concepts with Student Experience: Introducing Small-Molecule Crystallography to Chemistry Undergraduates Using a Flexible Laboratory Module
S.-L. Zheng, M.G. Campbell
J. Chem. Educ. 2018, 95, 2279–2283.
21
Fluoride Detection with a Redox-Active Naphthalene Diimide Metal–Organic Framework
H.C. Wentz, M.G. Campbell
Polyhedron 2018, 154, 309–313.
20
Argentophilic Interactions in Solution: An EXAFS Study of Silver(I) Nitrene Transfer Catalysts
C.L. Mak, B.C. Bostick, N.M. Yassin, M.G. Campbell
Inorg. Chem. 2018, 57, 5720–5722.
19
Metal–Organic Frameworks as Active Materials in Electronic Sensor Devices
M.G. Campbell, M. Dincă
Sensors 2017, 17, 1108.
MGC Publications from Work Prior to Barnard:
18
Isoreticular Linker Substitution in Conductive Metal‐Organic Frameworks with Through‐Space Transport Pathways
L. Xie, S.S. Park, M.J. Chmielewski, H. Liu, R.A. Kharod, M.G. Campbell, M. Dincă
Angew. Chem. Int. Ed. 2020, 59, 19623–19626.
17
Bridging the Gaps in 18F PET Tracer Development
M.G. Campbell, J. Mercier, C. Genicot, V. Gourverneur, J.M. Hooker, T. Ritter
Nature Chem. 2017, 9, 1–3.
16
Mechanistic Insight Into High-Spin Iron(I)-Catalyzed Butadiene Dimerization
H. Lee, M.G. Campbell, R.H. Sánchez, J. Börgel, J. Raynaud, S.E. Parker, T. Ritter
Organometallics 2016, 35, 2923–2929.
15
Teaching Outside the Classroom: Field Trips in Crystallography Education for Chemistry Students
B.J. Malbrecht, M.G. Campbell, Y.-S. Chen, S.–L. Zheng
J. Chem. Educ. 2016, 93, 1671–1675.
14
Teaching with the Case Study Method to Promote Active Learning in a Small Molecule Crystallography Course for Chemistry Students
M.G. Campbell, T.M. Powers, S.–L. Zheng
J. Chem. Educ. 2016, 93, 270–274.
13
Electrically Conductive Porous Metal–Organic Frameworks
L. Sun, M.G. Campbell, M. Dincă
Angew. Chem. Int. Ed. 2016, 55, 3566–3579.
12
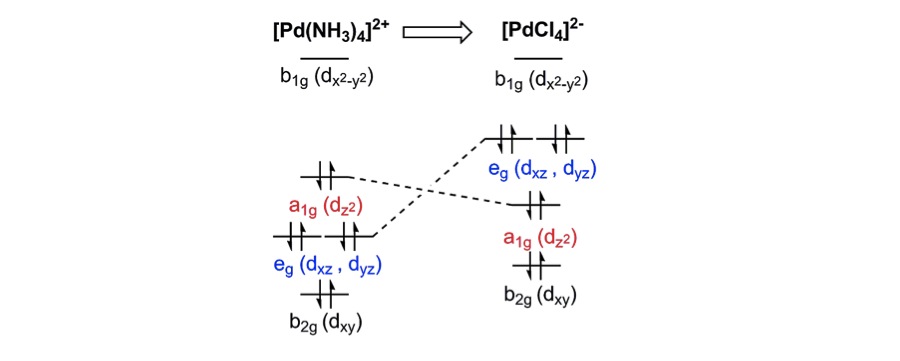
Transition Metal d-Orbital Splitting Diagrams: An Updated Educational Resource for Square Planar Complexes
J. Börgel, M.G. Campbell, T. Ritter
J. Chem. Educ. 2016, 93, 118–121.
11
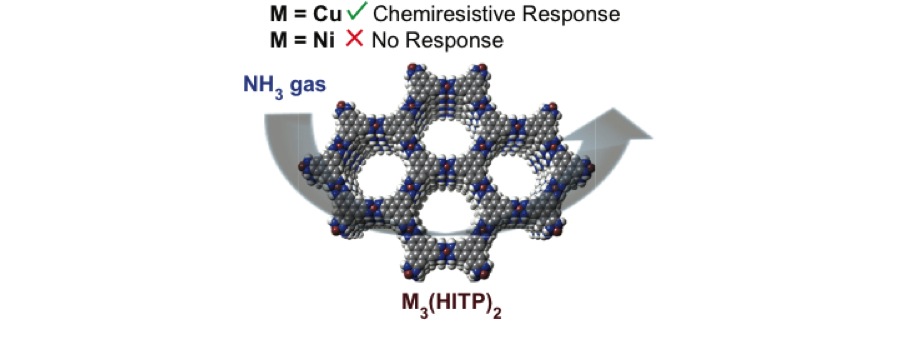
Chemiresistive Sensor Arrays from Conductive 2D Metal–Organic Frameworks
M.G. Campbell, S.F. Liu, T.M. Swager, M. Dincă
J. Am. Chem. Soc. 2015, 137, 13780–13783.
10
Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal–Organic Framework for Chemiresistive Sensing
M.G. Campbell, D. Sheberla, S.F. Liu, T.M. Swager, M. Dincă
Angew. Chem. Int. Ed. 2015, 54, 4349–4352.
9
Modern Carbon–Fluorine Bond Forming Reactions for Aryl Fluoride Synthesis
M.G. Campbell, T. Ritter
Chem. Rev. 2015, 115, 612–633.
8
Transition Metal-Mediated and Metal-Catalyzed Carbon–Fluorine Bond Formation
M.G. Campbell, A.J. Hoover, T. Ritter
Top. Organomet. Chem. 2015, 52, 1–54.
7
Late-Stage Formation of Carbon–Fluorine Bonds
M.G. Campbell, T. Ritter
Chem. Rec. 2014, 14, 482–491.
6
Late-Stage Fluorination: From Fundamentals to Application
M.G. Campbell, T. Ritter
Org. Process Res. Dev. 2014, 18, 474–480.
5
Support of academic synthetic chemistry using separation technologies from the pharmaceutical industry
E.L. Regalado, M.C. Kozlowski, J. Curto, T. Ritter, M.G. Campbell, A.R. Mazzotti, B. Hamper, C.D. Spilling, M.P. Mannino, L. Wan, J.-Q. Yu, J. Liu, C.J. Welch
Org. Biomol. Chem. 2014, 12, 2161–2166.
4
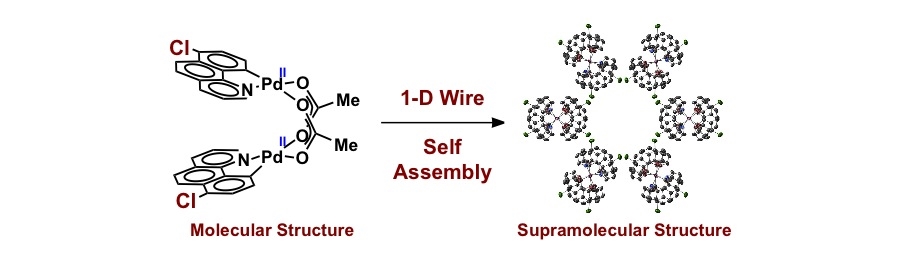
One-Dimensional Palladium Wires: Influence of Molecular Changes on Supramolecular Structure
M.G. Campbell, S.-L. Zheng, T. Ritter
Inorg. Chem. 2013, 52, 13295–13297.
3
Palladium(III)-Catalyzed Fluorination of Arylboronic Acid Derivatives
A.R. Mazzotti, M.G. Campbell, P. Tang, J.M. Murphy, T. Ritter
J. Am. Chem. Soc. 2013, 135, 14012–14015.
2
Synthesis and structure of solution-stable one-dimensional palladium wires
M.G. Campbell, D.C. Powers, J. Raynaud, M.J. Graham, P. Xie, E. Lee, T. Ritter
Nature Chem. 2011, 3, 949–953.
1
Reaction of acetylated carbohydrates with trimethylaluminum: concise synthesis of 1,2-O-isopropylidene D‑ribofuranose
J.D. More, M.G. Campbell
Tetrahedron Lett. 2009, 50, 2617–2619.